Prof. Ling co-authored article published in journal "Nature Sustainability"

09 Mar 2020

WTCR-Editor
Prof. Ling co-authored the new article published in journal “Nature Sustainability” - CO2 mineralization and utilization by alkaline solid wastes for potential carbon reduction. This paper summarizes the amount of carbon dioxide (CO2) absorbed and reduced by different types of alkaline solid wastes using accelerated carbonation in different regions of the world, and the global potential of CO2 emission reduction under this initiative is estimated.
(1) Background of carbon sequestration by alkaline solid wastes
The use of alkaline solid waste for CO2 mineralization and utilization is a new technology developed rapidly in the last decade. Globally, available alkaline solid wastes for CO2 mineralization and utilization include steel slag, coal slag, fuel combustion products, mining/mineral processing waste, incinerator waste, cement/concrete waste, pulp/paper mill waste, etc.
The calcium and magnesium oxide in these materials can be hydrated in water and react with CO2 flue gas at high pH (usually above 10) to form carbonates by accelerated carbonation reaction. The amount of CO2 absorbed by this measurement is counted as a direct CO2 reduction. Moreover, the solid waste after the reaction can be used as clinker to partially replace the raw materials or used as aggregate in concrete, which further reduce the exploitation of natural materials. This is counted as indirect CO2 reduction.
(2) Research on global capacity for direct and indirect CO2 reduction
The study on the global capacity for direct and indirect CO2 reduction shows that the potential for direct and indirect CO2 reductions is significant in Asia-pacific regions due to the high production of alkaline solid wastes. The figure below also shows that the use of carbonated steel slag products contributes more to the total CO2 reduction than carbonation processes (direct CO2 reduction). It is pointed out that the energy consumption of grinding, stirring, and using reactor during carbonation should be taken into account in the actual carbon sequestration, and the use of renewable resources such as solar photovoltaic energy can effectively reduce CO2 emissions.
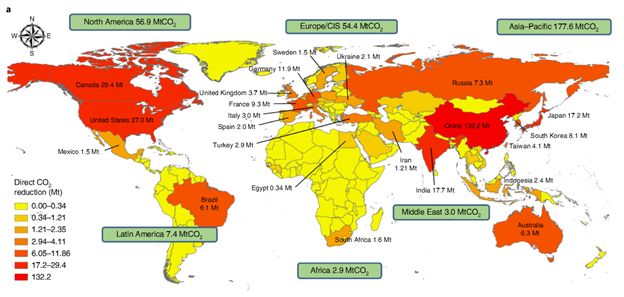
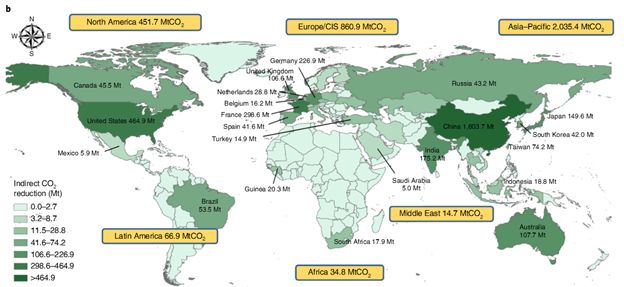
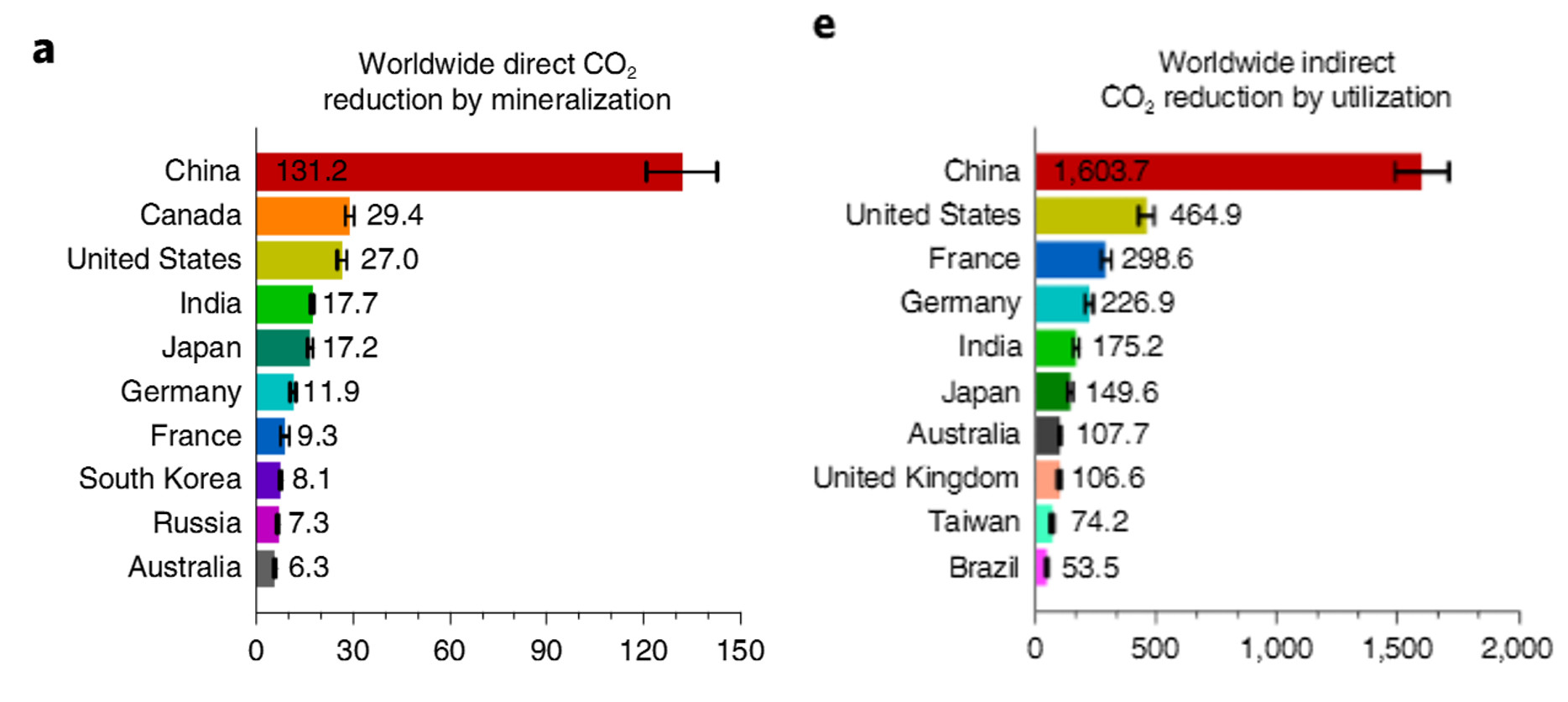
As figure 3 presents, China has the highest direct and indirect CO2 reduction globally, which is about four times of the second place.
(3) Study on the capability of direct and indirect CO2 reduction of different alkaline solid wastes
Figure below (left) states that 43.5% of the direct CO2 reduction can be achieved through the reuse of iron slag and steel slag, followed by cement/concrete waste (16.3%), mining/mineral processing waste (13.5%) and coal combustion waste (12.3%). The figure on the right shows that cement/concrete waste (55.7%) contributes the most to indirect CO2 reduction.
(3) Study on the capability of direct and indirect CO2 reduction of different alkaline solid wastes
Figure below (left) states that 43.5% of the direct CO2 reduction can be achieved through the reuse of iron slag and steel slag, followed by cement/concrete waste (16.3%), mining/mineral processing waste (13.5%) and coal combustion waste (12.3%). The figure on the right shows that cement/concrete waste (55.7%) contributes the most to indirect CO2 reduction.

(4) Conclusions
The amount of CO2 reduction calculated in this paper is ideal, and it depends on various political and social factors to a great extent, such as implementation of command and control (governance), provision of economic instruments, maturity of key mineralization technology, advancement of research and development programs, and establishment of information exchange platforms.
On a technical level, the feasibility of carbonation of alkaline solid wastes is influenced by a number of factors in different regions and areas:
a) The features of the CO2 source (e.g. availability and purity) and low-cost alkaline solid wastes (e.g. quantity and maximum achievable carbonation capacity);
b) The logistics of feedstock and products (e.g. distance between CO2 and waste source);
c) The reliability, scalability, and intergrability of key process element, such as reactors;
d) The required product specifications and its market viability.
As the indirect CO2 reduction is significantly higher than the direct CO2 reduction, relevant government policies, factories, and research directions can focus more towards the application of carbonated alkaline solid waste products.
File link:
https://www.nature.com/articles/s41893-020-0486-9.epdf?author_access_token=0nbZIHdndyS1EXxlmYYGMNRgN0jAjWel9jnR3ZoTv0NTbrmOVe47RMP3VJMqu3_px_1hd8pTPSUqhqXqJtCWZbfdvANG_AJpQs-XlOInzTOcMJfqs_XKaiIxJFGjpZP5OkP30fQY50Bsn5I8PL6HUw%3D%3D
The amount of CO2 reduction calculated in this paper is ideal, and it depends on various political and social factors to a great extent, such as implementation of command and control (governance), provision of economic instruments, maturity of key mineralization technology, advancement of research and development programs, and establishment of information exchange platforms.
On a technical level, the feasibility of carbonation of alkaline solid wastes is influenced by a number of factors in different regions and areas:
a) The features of the CO2 source (e.g. availability and purity) and low-cost alkaline solid wastes (e.g. quantity and maximum achievable carbonation capacity);
b) The logistics of feedstock and products (e.g. distance between CO2 and waste source);
c) The reliability, scalability, and intergrability of key process element, such as reactors;
d) The required product specifications and its market viability.
As the indirect CO2 reduction is significantly higher than the direct CO2 reduction, relevant government policies, factories, and research directions can focus more towards the application of carbonated alkaline solid waste products.
File link:
https://www.nature.com/articles/s41893-020-0486-9.epdf?author_access_token=0nbZIHdndyS1EXxlmYYGMNRgN0jAjWel9jnR3ZoTv0NTbrmOVe47RMP3VJMqu3_px_1hd8pTPSUqhqXqJtCWZbfdvANG_AJpQs-XlOInzTOcMJfqs_XKaiIxJFGjpZP5OkP30fQY50Bsn5I8PL6HUw%3D%3D





